
Harold H. Burdsall, Jr., Thomas J. Volk*, Center for Forest Mycology Research, Forest Products Laboratory *, Forest Service, United States Department of Agriculture, One Gifford Pinchot Dr., Madison, WI 53705 USA
& Joseph F. Ammirati, Jr., Department of Botany, KB-15, University of Washington, Seattle WA 98195 USA
*Current Address of T. Volk: Dept. of Biology and Microbiology, 3024 Cowley Hall, University of Wisconsin-La Crosse, La Crosse WI 54601
Originally published in Mycotaxon 60:387-395, 1996
See also some additional material at http://www.botany.wisc.edu/fungi/june97.html

ABSTRACT: The new genus Bridgeoporus is proposed to accommodate Oxyporus nobilissimus W.B. Cooke. Bridgeoporus is associated with a brown rot of wood, as opposed to the superficially similar genera Oxyporus and Rigidoporus, which are associated with white rot. Bridgeoporus lacks clamp connections at the septa, is monomitic, possesses pseudocystidia, and has a unique configuration of fascicles of hyphae making up the upper surface of its pileus. This combination of characters is found in no previously described genus.
* The Forest Products Lab is maintained in cooperation with the University of Wisconsin-Madison. This publication was written and prepared by U.S. government employees on official time, and it is therefore in the public domain and not subject to copyright.
INTRODUCTION
Oxyporus nobilissimus W.B. Cooke (Cooke, 1949) has attracted a great deal of attention in the past several years (Christy, 1991; Coombs, 1991) because of its large size, uncommon occurrence, unique appearance, and association with very large host trees. Commonly called the "Fuzzy Sandozi," it has been reported infrequently (WTU Herbarium records; Trappe, 1990; Christy, 1991) since its discovery in 1943. Currently, living specimens are known from only six sites in Oregon and Washington. The fungus produces perennial basidiomata that may weigh up to up to 130 kg and are usually associated with large diameter (at least 1 m) Abies procera Rehd., but occasionally with equally large Abies amabilis (Dougl.) Forb. or possibly Tsuga heterophylla (Raf.) Sarg. It is the first fungus to be listed as an endangered species by any private or public agency in the United States, having been listed as such by the Oregon Natural Heritage program (Christy, Pers. Comm.; Lizon, 1995). O. nobilissimus has also been listed by the Forest Ecosystem management team (FEMAT; anon., 1994) as one of 252 species of fungi that must be surveyed for in the habitat of the northern spotted owl.
The genus Oxyporus (Bourd. & Galz.) Donk accommodates species with a monomitic hyphal system, simple-septate hyphae, and causing white rot. Oxyporus nobilissimus has been reported to cause a brown rot (Cooke, 1949), but the species has been left in Oxyporus (e.g., by Gilbertson & Ryvarden, 1987) because of a lack of specific studies demonstrating the type of rot. It has also been placed in Fomes (Fr.) Fr. (Lowe, 1955). However, because of the following combination of characteristics, i.e. simple-septate hyphae, association with brown rot, pseudocystidia of tramal origin, and closely-appressed hyphae in fascicles making up the upper surface of its pileus, it is being placed in a new genus, Bridgeoporus.
METHODS AND MATERIALS
Relatively small (5-6 cm) portions of basidiomata were carefully excised from living specimens and cultured according to Gilbertson & Ryvarden (1986) on 1.5% malt extract 2% agar, plus 2 mg/l benomyl and 100 mg/l streptomycin. Microscopic characters of the basidiomata were determined by mounting thin sections in 3% KOH plus 1% phloxine or in Melzer's reagent (Gilbertson & Ryvarden, 1986). Line drawings were made with the aid of a camera lucida and drawing tube. Putative cultures and portions of basidiomata have been preserved at CFMR, Madison, Wisconsin. Herbarium abbreviations are as in Holmgren et al. (1990). Colors are as in Ridgway (1912). PCR amplification and sequencing was performed according to Nakasone & Sytsma (1993).
RESULTS AND DISCUSSION
Although collected basidiospores failed to germinate, we collected a number of putative cultures from tissues of four basidiomata and from the brown-rotted wood. We amplified and sequenced the internal transcribed spacer (ITS) region of the ribosomal DNA repeat from DNA obtained from basidiospore drops and from putative cultures; the DNA sequence from the spore drops (two samples) and 3 putative cultures did not match. Thus we could not positively conclude that any of the tissue cultures were that of Oxyporus nobilissimus. However, O.nobilissimus has been reported to cause a brown rot because of the decay observed in the wood attached to the type specimen (Cooke, 1949). Our observations of wood in association with living specimens (whenever possible) also indicate brown rot. We are confident that O.nobilissimus causes a brown rot. The brown rot associated with the basidiomata is in contrast to that of the type species of Oxyporus, Polyporus connatus Weinm. [ = Polyporus populinus Schum.:Fr., = Oxyporus populinus (Schum.:Fr.) Donk ], which causes a white rot.
Besides the difference in rot characteristics, which are considered important at the generic level (e.g. Nobles, 1958; Donk, 1960; Ryvarden, 1991), Oxyporus (Bourd. & Galz.) Donk differs from O.nobilissimus in having true cystidia that arise from the subhymenium rather than pseudocystidia, which are differentiated hyphal end cells that arise in the trama. Species of Rigidoporus Murr. are similar to O.nobilissimus in possessing pseudocystidia, similar basidiospore and basidium shape and size, and both thin- and thick-walled generative hyphae, but it causes a white rot. Rigidoporus is probably the closest genus for placement of this species, but its members cause a white rot and lack the unique pileus surface hyphal structure of O.nobilissimus. Lowe (1955) placed O.nobilissimus in Fomes (Fr.) Fr. at a time when that genus encompassed a wide variety of large, conk-shaped species. It is no longer an appropriate placement since the concept of Fomes has been narrowed to include only trimitic species, with clamped generative hyphae and sclerids, and causing a white rot.
Other genera with simple-septate hyphae that we considered for placement of this species included Physisporinus Karst., but its members have resupinate, soft, waxy basidiomata and are associated with a white rot. Pycnoporellus Murr., em. Kotl. & Pouz., contains species that cause a brown rot and form bright orange to rust colored basidiomata that turn deep red in KOH. Postia Fr. (= Oligoporus Bref.) contains species whose members have clamp-connections on generative hyphae. In addition, the broadly ovoid basidiospore shape in O.nobilissimus is very different from known Postia species. None of the above genera contain species with the unique hyphal structure of hyphae on the pileus surface that is found in O.nobilissimus. Since no described genus is appropriate, we propose a new genus, Bridgeoporus, to accommodate this unique species.
Bridgeoporus Volk, Burdsall & Ammirati gen. nov. Figs. 1-8. Basidiomatibus perennis, effusis-reflexis vel substipitatis, imbricatis, concrestis, suberosis, lignosis; pileis dense strigosis, fibris ramosis hispidis, zonatis; systemate hyphae monomitico, hyphis hyalinis, efibulatis; pseudocystidiis hyalinis; basidiosporis hyalinis, laevibus, nonamyloideis. Consociateus una cum tabes brunneo.
Etymology: Bridgeoporus m. Named for William Bridge Cooke (widely known as "Bridge"), who described Oxyporus nobilissimus.
Type species: Bridgeoporus nobilissimus (W.B. Cooke) Volk, Burdsall, & Ammirati comb. nov. basionym: Oxyporus nobilissimus W.B. Cooke, Mycologia 41: 444 (1949) Fomes nobilissimus (W.B. Cooke) Lowe, Mycologia 47:219 (1955)

 Basidiomata perennial, 30-140 x 25-95 x 30-100 cm, sessile, ungulate, imbricate, or centrally substipitate, with the form depending on location on host tree (snag, stump, or living) (Fig. 1, 2) often occluding small twigs and other objects . Texture fibrous, rubbery and tough when fresh.
Basidiomata perennial, 30-140 x 25-95 x 30-100 cm, sessile, ungulate, imbricate, or centrally substipitate, with the form depending on location on host tree (snag, stump, or living) (Fig. 1, 2) often occluding small twigs and other objects . Texture fibrous, rubbery and tough when fresh.
 Pileus surface (Fig. 3) a dense mat of white mycelial fibers in youth, often somewhat agglutinated at the tips, becoming cinnamon brown or darker in age and usually reaching several mm in length, often appearing green due to epiphytic association with several species of unicellular algae. Context up to 1.5 cm thick, white, tough, rubbery, and fibrous when fresh, cinnamon-buff to ochraceous, hard and brittle when dried. Pores concolorous with context, round, 2 per mm, 2-7 mm long in mature layers, not becoming stuffed, stratified, with a layer of sterile tissue 2-3 mm thick between successive pore layers.
_________________________________________
Pileus surface (Fig. 3) a dense mat of white mycelial fibers in youth, often somewhat agglutinated at the tips, becoming cinnamon brown or darker in age and usually reaching several mm in length, often appearing green due to epiphytic association with several species of unicellular algae. Context up to 1.5 cm thick, white, tough, rubbery, and fibrous when fresh, cinnamon-buff to ochraceous, hard and brittle when dried. Pores concolorous with context, round, 2 per mm, 2-7 mm long in mature layers, not becoming stuffed, stratified, with a layer of sterile tissue 2-3 mm thick between successive pore layers.
_________________________________________
Figs. 1-3. (above) Macroscopic characters of Bridgeoporus nobilissimus. 1. Sessile form growing on the side of a snag. Note brown rotted wood behind the somewhat inconspicuous specimen in the center. 2. Centrally stipitate form growing on the ground from roots of a snag. 3. Closeup of pileus surface with coarse hairs. For information on how to obtain color computer images of these and other photographs of B.nobilissimus please contact T.Volk, whose current e-mail address is volk.thom@uwlax.edu
Fibers of the pileus surface 10-30 µm X (50)-60-75 µm, with frequent branching and anastomosing, showing layering as evidence of seasonally added increments of growth, new growth of the fibers occurring in discrete tufts of agglutinated hyphae scattered over the pileus surface, especially near the margin. The fibers are composed of bundles of simple-septate, parallel hyphae, 2-3 µm diam, hyaline to yellow-brown, simple-septate, thin- to slightly thick-walled, infrequently branching.
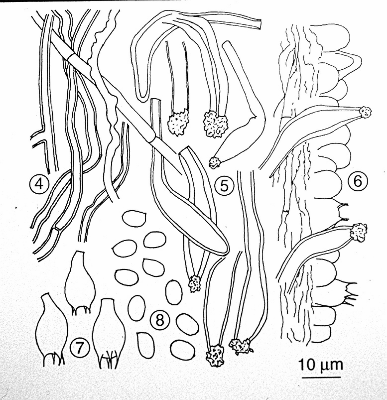 Figs. 4-8. Microscopic characters of Bridgeoporus nobilissimus. Fig. 4. Context hyphae. 5. Pseudocystidia. 6. Hymenium. 7. Basidia. 8. Basidiospores.
Figs. 4-8. Microscopic characters of Bridgeoporus nobilissimus. Fig. 4. Context hyphae. 5. Pseudocystidia. 6. Hymenium. 7. Basidia. 8. Basidiospores.
Habitat: Occurring singly or occasionally imbricate on old growth Abies procera (1-2 m or more diameter at breast height), rarely Abies amabilis or possibly Tsuga heterophylla. Position on the host is variable. It has been found near the root collar of living trees or snags, on sides of snags not far from the ground (1 m maximum height), and on old cut stumps, but not known to occur on downed logs.
Distribution: Known only from the Cascade Mountain Range in Washington and Oregon and the Coast Range on the Olympic Peninsula in Washington. Locations where basidiomata are found were given letter designations (e.g. AC, GM, LM, SP, HT, NR) based on their location. Number in parentheses following the location indicate number of basidiomata found at that site. Since these are perennial basidiomata of rarely-found fungi that are still extant, we decline to give exact locations in order to protect them.
Specimens examined: A.H. Smith No. 31102, holotype MICH, isotype WTU, Mt. Rainier National Park, WA, Abies sp., 1948; Extant basidiomata (portions of which are at CFMR):, AC (3), King Co., Mt. Baker-Snoqualmie National Forest, WA, A.procera, 1992; GM (4) Cowlitz Co., Mt. St. Helens National Monument, WA, A.procera, 1992; LM (6) Multnomah Co., Mt. Hood National Forest, OR, A.procera, T.heterophylla, 1992, 1994; SP (15) Linn Co., Bureau of Land Management, OR, A.procera, 1991, 1992; HT (2) Olympic National Forest, Gray's Harbor Co., WA, Abies amabilis, 1992, 1993; NR (2) Mt. Rainier National Park, Pierce Co., WA, A.procera, 1992.
REMARKS: The type specimen was reported on Tsuga heterophylla (Cooke, 1949). However, microscopic examination of the wood attached to the base of the isotype is that of an Abies sp. (Regis Miller, Center for Wood Anatomy Research, Forest Products Lab, Personal Communication). This is consistent with the vast majority of specimen/host associations noted for B. nobilissimus. However one of the specimens in Multnomah County has been found at the base of a large T. heterophylla snag next to a large Abies procera snag. The host association of this specimen is unclear.
Cooke (1949) considered the fungus to have a dimitic hyphal system. Although thin-walled and thick-walled hyphae are present in the basidiomata, the latter should not be considered skeletal hyphae because of their abundant septa, but rather as sclerified generative hyphae. We concur with Gilbertson & Ryvarden (1987) who report O. nobilissimus as monomitic. Additional illustrations and descriptions of micro- and macroscopic characteristics can be found in Lowe (1955, 1957), Gilbertson & Ryvarden (1987), Coombs (1991) and Christy (1991).
The mat of mycelia on the upper surface is not solid, but very porous, containing cracks and crevasses that allow the accumulation of water and organic debris. Unicellular algae, including Coccomyxa sp. and Charicium spp., also occur. Our studies indicate that the algae appear to be living among fungal hyphae and not endophytically. In addition, some vascular plants (e.g. Oxalis sp. and pteridophytes) and bryophytes sometimes occur in and on the surface of the pileus.
As indicated in the description, a layer of external hyphae covers the previous year's pore surface before the initiation of a new pore layer. Each new layer arises from coalescence of discrete patches of mycelium formed external to the old pores. The pores begin as indentations on the layer of confluent sterile hyphae.
ACKNOWLEDGMENTS: We thank James Trappe, as well as Don H. Coombs and M. Maggie Rogers (the editors of Mushroom, the Journal of Wild Mushrooming) for renewing interest in this fungus. We thank Glenn Walker, James H. Ginns, David Shaw, Claire Hibler, Judy Roger, Lorelei Norvell, Elisabeth Eyestone, and Don and Bonnie Grandorff for help in finding and collecting specimens and putative cultures of this fungus. We thank Karen K. Nakasone, Rita M. Rentmeester and Kelly Collins for testing the identity of the cultures using PCR fingerprinting and DNA sequencing. We thank Linda and James Graham for identification of algal associates and Regis Miller for identification of host tree species. We thank Karen K. Nakasone and Michael J. Larsen for help with the Latin diagnosis. We thank Michael J. Larsen and Robert L. Gilbertson for review of the manuscript. We also thank Erast Parmasto, Leif Ryvarden, and Tuomo Niemelš for helpful discussions on the proper placement of this species.
LITERATURE CITED
Anonymous. 1994. Standards and Guidelines for management of habitat for late-successional and old-growth forest related species within the range of the northern spotted owl. Attachment A to the Record of decision for amendments to Forest Service and Bureau of Land Management planning documents within the range of the northern spotted owl. US Government Printing Office, Region 10.
Christy, J. 1991. The most noble polypore--- Endangered or already gone to passenger pigeon-land. Mushroom, the Journal of Wild Mushrooming 9(3):11
Cooke, W.B. 1949. Oxyporus nobilissimus and the genus Oxyporus in North America. Mycologia 41:442-455.
Coombs, D.H., 1991. Looking for a big fuzzy one. Mushroom, the Journal of Wild Mushrooming 9 (4):5-8
Donk, M. 1960. The generic names proposed for Polyporaceae. Persoonia 1:173-302.
Gilbertson, R.L. and L. Ryvarden. 1986. North American Polypores. Vol. 1 Abortiporus-Lindtneria. Oslo: Fungiflora. pp. 1-436.
Gilbertson, R.L. and L. Ryvarden. 1987. North American Polypores. Vol. 2. Megasporoporia-Wrightoporia. Oslo: Fungiflora. pp. 437-885.
Holmgren, P.K., N.H. Holmgren, and L.B. Barnett. 1990. Index Herbariorum. Eighth Edition. New York Botanical Garden. 693 pp.
Lizon, P. 1995. Preserving the biodiversity of fungi. Inoculum 46 (6): 1-4.
Lowe, J.L., 1955. Perennial polypores of North America III. Fomes with context white to rose. Mycologia 47:213-224
Lowe, J.L., 1957. Polyporaceae of North America: The genus Fomes. State University College of Forestry at Syracuse University, Technical Publication No. 80. 97 pp.
Nakasone, K.K. and K.J. Sytsma. 1993. Biosystematic studies on Phlebia acerina, P.rufa, and P.radiata in North America. Mycologia 85:996-1016.
Nobles, M.K. 1958. Cultural characters as a guide to the taxonomy and phylogeny of the Polyporaceae. Canad. J. Bot. 36:883-926
Ridgway, R. 1912. Color Standards and Color Nomenclature. 53 plates.
Ryvarden L. 1991. Genera of Polypores: Nomenclature and Taxonomy. Fungiflora, Oslo, Norway. Synopsis Fungorum 5. 363 pp.
Trappe, J.M. 1990. The "most noble" polypore endangered. In Ancient Forests of the Pacific Northwest. Elliot A Norse. The Wilderness Society. Island press. Washington DC pp. 126-127.